Lithium Surface Treatment for Refractory Metal Plasma Facing Components (PFCs) for Fusion Power Systems
PFCs need to survive a very extreme environment inside a tokomak (fusion reactor vessel): such as high temperatures, large changes in temperature, and bombardment by ions. To survive these conditions, materials are likely to be refractory materials (e.g. tungsten (W), molybdenum (Mo), etc.) with tolerance to temperature extremes in a fusion environment. However, these metals suffer damage from incident ions from the fusion plasma. A possible solution to the ion damage is a Li surface treatment. This surface treatment could protect the PFCs from ions as well as improve the performance of the Fusion plasma.
Highlight: Method of Deuterium Retention in Lithium Surface Treatment
The use of Li as a surface treatment on plasma facing components in tokamaks has improved the performance of fusion plasmas. The reason for this improvement is that Li controls the release of hydrogen fuel isotopes–deuterium (D) and tritium (T) back into the fusion plasma. On graphite, this is the result of bonds between lithium, oxygen (O), and deuterium. This behavior is also observed on W. We have been able to observe this behavior using in situ surface chemistry analysis using x-ray photoelectron spectroscopy (XPS).
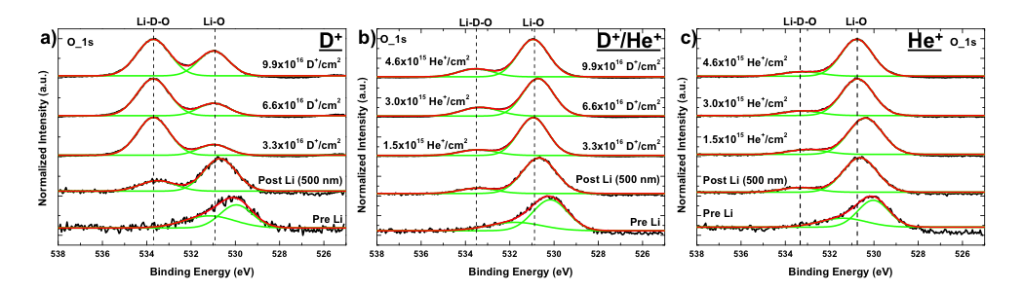
These surface chemistry studies of Li coatings on both graphite and W show a formation of a new binding functionality (peak) when it is exposed to D ions. An example of the W case is presented in the figure below. This peak is associated with the retention of hydrogen. Computer simulations investigated which atoms prefer to bond to which of its closest neighbors. The model showed that the Li prefers to bond to O and that the D prefers to bond to the O. Therefore, it is the O that retains the D but Li is required to trap the O.
Another aspect of this retention, that we are investigating, is if it can be controlled. When helium (He) was added to the exposure of the Li coating on W, to simulate the fusion products, the functionality observed with D exposure is reduced as seen in the figure below. By changing the percentage of He, we may be able to control how well the Li retains the D.
Oxygen X-ray Photoelectron Spectroscopy scans of Li coatings on W exposed to a) D, b) D/He, and c) He ions with increasing dose. These scans show a decrease in the peak associated with the retention of D when irradiated with D.
Sponsors
Department of Energy
Selected Publications
– P. S. Krstic, et al. Deuterium uptake in magnetic- fusion devices with lithium-conditioned carbon walls. Physical Review Letters, 110(10):105001, March 2013.
People